Sugita Titanium Aneurysm Clip II (T2) & Appliers|Aneurysm Clips|products|MizuhoMedical consistently strives to be the leading worldwide provider of medical products
Products
- HOME >
- Products >
- Aneurysm Clips >
- Sugita Titanium Aneurysm Clip II (T2) & Appliers
Operating Table
Neurosurgery
Surgical Instruments
NIR Imaging
Aneurysm Clips
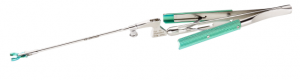
Sugita Titanium Aneurysm Clip II (T2) & Appliers
Sugita Titanium aneurysm Clip Ⅱ from Mizuho.
➡ here: catalog DL
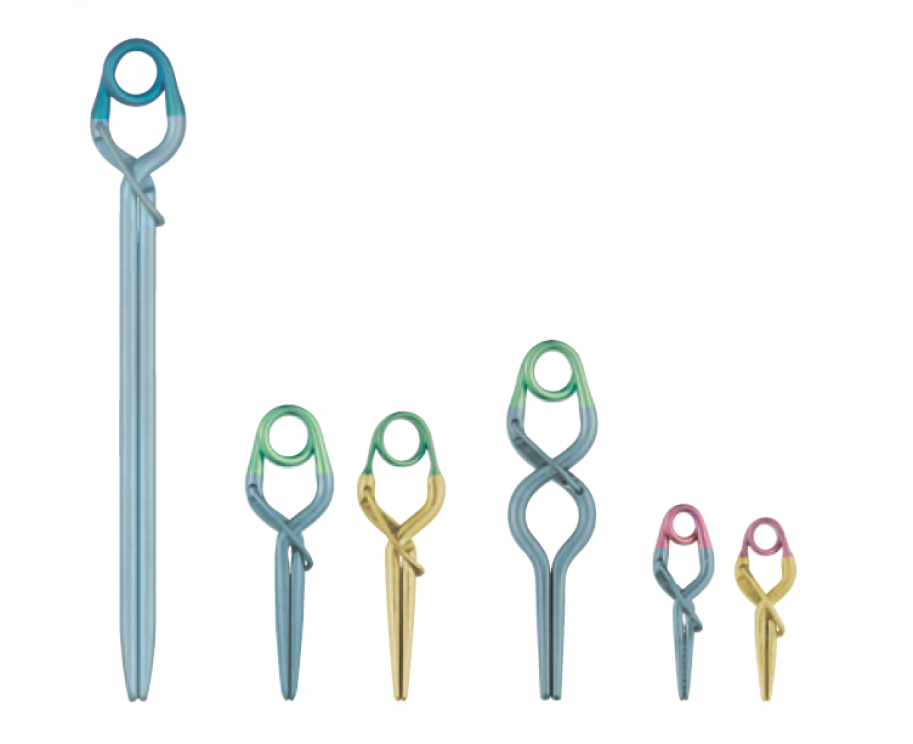
- Slim Spring
- Lower-profiled spring than Sugita Aneurysm Clip.
- Tapered Blade
- A slim blade design with tapered tip.
- Wide Opening
- Wider blade opening than conventional Sugita Titanium Clips.
- MRI Compatibility
- Non-clinical testing demonstrated that this clip is MR Conditional. In non-clinical testing, the image artifact caused by this clip extends approximately 5-mm from this clip when imaged using a gradient echo pulse sequence and a 3-Tesla MR system. (For more details, please refer to the IFU.)
- Single Shaft Applier
- For superior visibility and performance.
・Ultra low profile allows optimal visibility of operative site
・Support precise placement of Sugita titanium Aneurysm Clip II(T2)
・Meticulously milled jaw grooves help secure large and mini clip sizes
- MR safety information
- MR Conditional
Non-clinical testing demonstrated that this clip is MR Conditional. A patient with this clip can be scanned safely in an MR system under the following conditions:
• Static magnetic field of 1.5 Tesla or 3 Tesla, only.
• Maximum spatial gradient field of 4,000 Gauss/cm (40 Tesla/m)
• Maximum MR system reported, whole body averaged specific absorption rate (SAR) of 2 W/kg for 15 minutes of scanning (i.e. per pulse sequence) in the Normal Operating Mode)
Under the scan conditions defined, this clip is expected to produce a maximum temperature rise of 2.2°C after 15 minutes of continuous scanning (i.e. per pulse sequence)
In non-clinical testing, the image artifact caused by the clip extends approximately 5 mm from this clip when imaged using a gradient echo pulse sequence and 3 Tesla MR system.
NOTE: Do not take the Sugita Clip Applier into the MR environment. This device contains material which has been determined to be MR Unsafe.